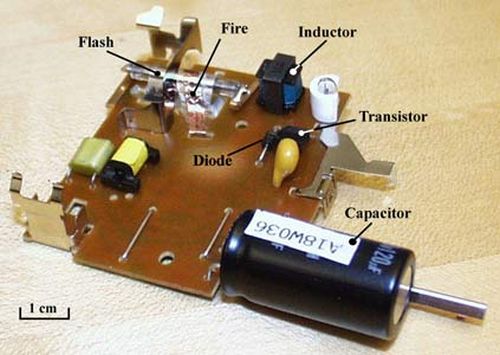
Making a Flash
A basic camera flash system, like you would find in a point-and-shoot camera, has three major parts.
- A small battery, which serves as the power supply
- A gas discharge tube, which actually produces the flash
- A circuit (made up of a number of electrical components), which connects the power supply to the discharge tube
The two components on the ends of the system are very simple. When you hook up a battery’s two terminals to a circuit, the battery forces electrons to flow through the circuit from one terminal to the other. The moving electrons, or current, provides energy to the various things connected to the circuit (see How Batteries Work for more information).
The discharge tube is a lot like a neon light or fluorescent lamp. It consists of a tube filled with xenon gas, with electrodes on either end and a metal trigger plate at the middle of the tube.
The basic idea is to conduct electrical current — to move free electrons — through the gas in the tube, from one electrode to the other. As the free electrons move, they energize xenon atoms, causing the atoms to emit visible light photons (see How Light Works for details on how atoms generate photons).
You can’t do this with the gas in its normal state, because it has very few free electrons — that is, nearly all the electrons are bonded to atoms, so there are almost no charged particles in the gas. To make the gas conductive, you have to introduce free electrons into the mix.
This is the metal trigger plate’s job. If you briefly apply a high positive voltage (electromotive force) to this plate, it will exert a strong attraction on the negatively charged electrons in the atoms. If this attraction is strong enough, it will pull the electrons free from the atoms. The process of removing an atom’s electrons is called ionization.
The free electrons have a negative charge, so once they are free, they will move toward the positively charged terminal and away from the negatively charged terminal. As the electrons move, they collide with other atoms, causing these atoms to lose electrons as well, further ionizing the gas. The speeding electrons collide with xenon atoms, which become energized and generate light (see How Fluorescent Lamps Work for more information).
To accomplish this, you need relatively high voltage (electrical “pressure”). It takes a couple hundred volts to move electrons between the two electrodes, and you need a few thousand volts to introduce enough free electrons to make the gas conductive.
For more detail: How Camera Flashes Work